Part 2: Testing the Release of Carbon Dioxide
EXPERIMENT #2
The second way in which the carbon dioxide can escape into the atmosphere is by being converted into oxygen and then being released through the hole in the plant compartment of the filtering system. To test how much oxygen is being made, a closed container with the potted English Ivy and a carbon dioxide meter will be exposed to pure carbon dioxide from the carbon dioxide tank. The closed container would not allow for the escape of carbon dioxide so the only way that the carbon dioxide levels would drop is if the carbon dioxide is being converted into oxygen through photosynthesis by the plants. Over a duration of time (ten minutes), the carbon dioxide levels can be monitored. The data will indicate the change in the carbon dioxide levels and the production of oxygen, which can be used to calculate the rate of conversion and the efficiency of the carbon dioxide-to-oxygen conversion.
Below is a video demonstrating the second experiment in part two of finding the conversion efficiency.
RESULTS:
This data indicates that the average air exchange for the system is decreasing. Sensor 1 has an air exchange rate of -0.7146 ppm CO2/hour and Sensor 2 has a value of -0.73107 ppm CO2/hour. This shows that the amount of carbon dioxide is decreasing over time in a closed system, as expected, because some of the carbon dioxide released is converted into something else (possibly oxygen).
The final scaled prototype that will be present as a visual is complete.
This week, experimentation has been done to measure air exchange rate for determining the efficiency of English ivy to eliminate CO2 from the atmosphere. The first test was done by monitoring CO2 using two sensors in a closed container with only an opening for CO2. Through that opening, CO2 was inserted and quickly closed. The entire container was also sealed off so that there was no air exchange with the outside environment. The concentration of CO2 over time was monitored and then used in an equation to calculate air exchange. The equation we used was as follows:


Below is a video demonstrating the second experiment in part two of finding the conversion efficiency.
RESULTS:
This data indicates that the average air exchange for the system is decreasing. Sensor 1 has an air exchange rate of -0.7146 ppm CO2/hour and Sensor 2 has a value of -0.73107 ppm CO2/hour. This shows that the amount of carbon dioxide is decreasing over time in a closed system, as expected, because some of the carbon dioxide released is converted into something else (possibly oxygen).
---------------------------------------------------------------------------
The final scaled prototype that will be present as a visual is complete.
-------------------------------------------------------------------------------------------------------
EXPERIMENTAL DATA
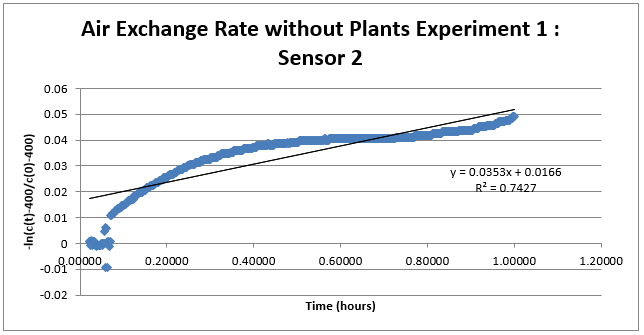
This week, experimentation has been done to measure air exchange rate for determining the efficiency of English ivy to eliminate CO2 from the atmosphere. The first test was done by monitoring CO2 using two sensors in a closed container with only an opening for CO2. Through that opening, CO2 was inserted and quickly closed. The entire container was also sealed off so that there was no air exchange with the outside environment. The concentration of CO2 over time was monitored and then used in an equation to calculate air exchange. The equation we used was as follows:


Using this formula, we were able to plot the data versus time to get a line equation of the rate of air exchange without plants. Two experiments were done for each sensor. Using the equation of the lines of each, the slope was determined and averaged for each sensor to get an average rate exchange for each sensor. The average rate for sensors 1 and 2 were .09020 ppm CO2/ hour and .06435 ppm CO2/hour, respectively. The data is shown below:
------------------------------------------------------------------------------------------------
Block Diagram for Attaining 10% Conversion Efficiency (Carbon Dioxide to Oxygen)



.jpg)